Abstract
Background
Consolidation with autologous stem cell transplantation (ASCT) was recommended for most responders to the initial peripheral T-cell lymphoma (PTCL) treatment. Evidence came primarily from retrospective studies affected by a selection bias between patients allocated to receive ASCT or not and a few prospective studies with no control groups (d'Amore F,2012; Ellin F, 2014). The National Comprehensive Cancer Network and the American Society for Transplantation and Cellular Therapy (ASTCT) recommend ASCT for patients with first-time complete remission (CR1). The European Society of Medical Oncology (ESMO) guidelines suggest that patients with first-time partial remission (PR1) could also be considered for ASCT (d'Amore F, 2015). However, the role of ASCT in patients with PTCL after CR1 or PR1 remains controversial. The COMPLETE study showed an insignificant tendency for better survival following ASCT in the PTCL with CR1 group (Park SI, 2016). The LYSA multicenter study investigated the effect of ASCT in patients with CR1 or PR1. Neither propensity score matching (PSM) nor Cox multivariate analysis supported the advantage of ASCT in upfront consolidation (Fossard G, 2018). However, well-designed randomized controlled trials to investigate the precise effect of ASCT in the front-line setting are lacking. Therefore, we aimed to investigate the efficacy of ASCT for PTCL with CR1 and PR1 by PSM analysis.
Methods
We pre-screened 8 826 patients with lymphoma admitted to Peking University, Cancer Hospital & Institute from January 1996 to November 2020. Patients with mature T/NK cell lymphomas were selected (n = 1 136). We excluded patients with NK/TCL or missing clinical data. Response assessment to the initial treatment was evaluated by 18F-FDG positron emission tomography/computed tomography (PET/CT) or CT using the Lugano criteria. We identified 685 patients with PTCL, of which 622 achieved CR1 or PR1. PSM was used to balance clinical indicators between the ASCT and non-ASCT groups. The primary endpoints were event-free survival (EFS) and overall survival (OS).
Results
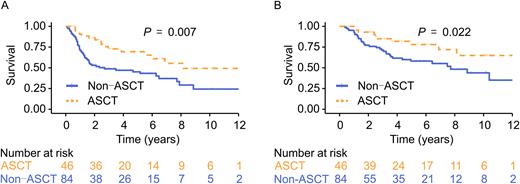
Among the 622 patients who underwent initial therapy, 272 (43.7%) achieved CR1, and 147 (23.6%) achieved PR1 after the treatment. After excluding 5 patients with subsequent unknown schemes following the initial treatment, 414 were included in the final analysis (ASCT, n = 73; non-ASCT, n = 341). With a median follow-up of 5.7 years for the entire cohort, long-term survival in the ASCT group was better than in the non-ASCT group (median EFS, 8.1 vs. 2.8 years, P = 0.002; median OS, 14.9 vs. 10.2 years; P = 0.007). The 5-year EFS and OS were 66.7% (95% CI, 56.0-79.4%) and 74.7% (95% CI, 64.1-87.0%) in patients with ASCT consolidation, and 26.6% (95% CI, 23.0-30.8%) and 43.6% (95% CI, 39.2-48.4%) in patients without ASCT. After PSM, the ASCT and non-ASCT groups were similar in their disease subtypes, LDH level, Ann Abor stage, and the response to the initial treatment. The survival advantage for ASCT (n = 46), as a consolidation procedure, over non-ASCT (n = 84; median EFS. 8.1 vs 2.7 years, P = 0.007; median OS, not reached vs 7.7 years; P = 0.022; Figure 1) was maintained even after PSM. For the entire cohort, ASCT was associated with better long-term survival than non-ASCT [hazard ratio (HR) for EFS, 0.46 (95% CI, 0.28-0.76), P = 0.003; HR for OS, 0.50 (95% CI, 0.31-0.84), P = 0.008)]. Subgroup analysis showed that patients at a higher risk based on International Prognostic Index (IPI) stratifications and advanced stages might benefit significantly from ASCT. Patients with angioimmunoblastic T-cell lymphoma (AITL) in the ASCT group appeared to show better survival than those in the non-ASCT group (EFS: HR, 0.26; 95% CI, 0.10-0.66; P = 0.005; OS: HR, 0.29; 95% CI, 0.12-0.74; P = 0.009). However, ASCT endowed no survival advantage for other PTCL subtypes.
Conclusion
ASCT could improve the long-term survival of responders to the initial treatment among patients with PTCL, especially those with AITL. However, the specific population that could benefit most from up-front ASCT needs to be characterized further.
Figure 1. EFS (A) and OS (B) after propensity score matching in the ASCT and non-ASCT groups
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal